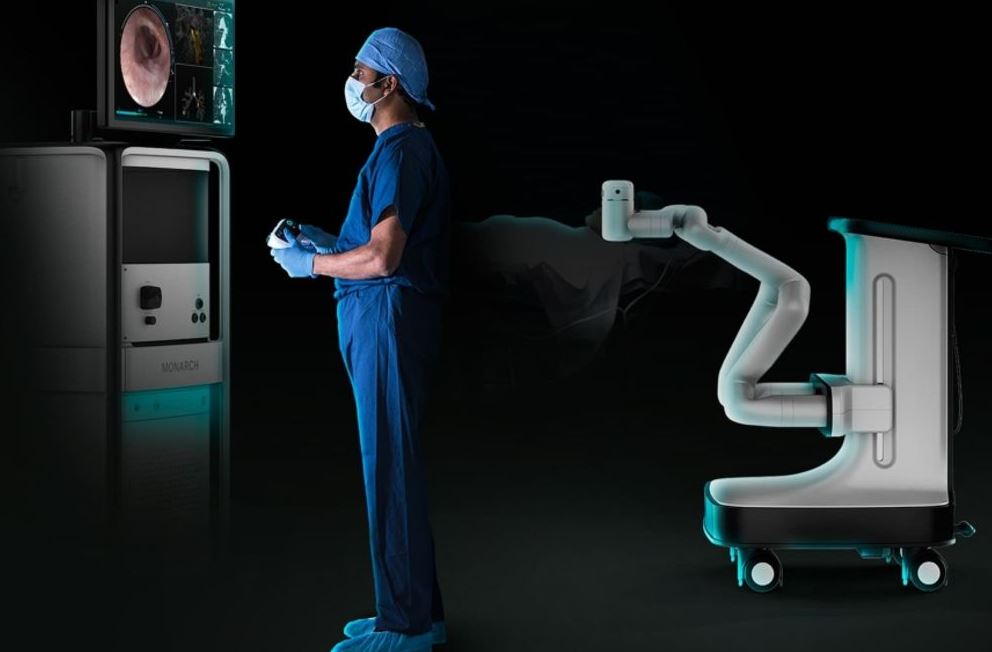
Ethicon, part of the Johnson & Johnson Medical Devices Company, announced the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for transbronchial microwave ablation technology using robotic-assisted bronchoscopy, which is currently under development.
The Breakthrough Devices Program is a voluntary program for certain medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. The goal of the Breakthrough Devices Program is to provide patients and health care providers with timely access to these medical devices by speeding up their development, assessment, and review, while preserving the statutory standards for premarket approval, 510(k) clearance, and De Novo marketing authorization, consistent with the Agency’s mission to protect and promote public health.
“Our acquisitions of NeuWave Medical and Auris Health have enabled us to bring two best-in-class platforms together as we work to develop this breakthrough technology,” said Vladimir Makatsaria, Company Group Chairman of Ethicon, Johnson & Johnson. “We look forward to working collaboratively with the FDA to help prioritize development and access for patients.”
Ethicon is a global leader in soft tissue microwave ablation and in flexible endoluminal robotics. The NEUWAVE Microwave Ablation System provides a minimally invasive option for soft tissue lesions, with more than 45,000 procedures performed to date. The MONARCH Platform provides improved reach into the periphery of the lung with continuous real-time vision, precision and control. MONARCH was the first robotic-assisted bronchoscopy system introduced in the United States with more than 3,300 procedures performed to date.
“Through our commitment to transform patients’ lives, Johnson & Johnson is advancing innovative solutions with a focus on the prevention, interception and cure of some of the world’s most complex, life-threatening diseases,” said Avrum Spira, M.D., M.Sc., Global Head of the Lung Cancer Initiative at Johnson & Johnson. “This promising convergence of technologies offers an exciting opportunity to catalyze new approaches and solutions to improve patient outcomes, and we look forward to evaluating this device in a comprehensive clinical development program.”
See Full Press Release at the Source: U.S. FDA Grants Ethicon Breakthrough Device Designation for Monarch-enabled NeuWave Microwave Ablation Technology
Press Release by: Ethicon
 Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.