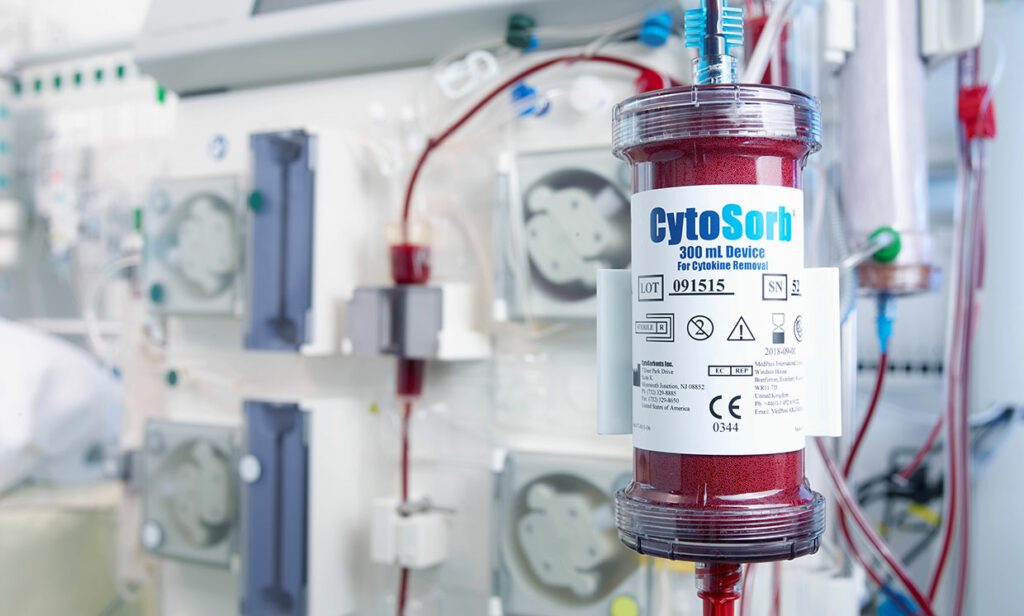
CytoSorbents Corporation, a critical care immunotherapy leader commercializing its CytoSorb® blood purification technology to treat cytokine storm and deadly inflammation in critically-ill and cardiac surgery patients around the world, announced the United States Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) of CytoSorb® for use in patients with COVID-19 infection.
Under the EUA, CytoSorbents can make CytoSorb available, through commercial sales, to all hospitals in the United States for use in patients, 18 years of age or older, with confirmed COVID-19 infection who are admitted to the intensive care unit (ICU) with confirmed or imminent respiratory failure who have early acute lung injury or acute respiratory distress syndrome (ARDS), severe disease, or life-threatening illness resulting in respiratory failure, septic shock, and/or multiple organ dysfunction or failure, as described in FDA’s authorization and as detailed below.
Patients with COVID-19 infection often exhibit a cytokine storm with severe hyperinflammation that can contribute to worsened injury to vital organs like the lungs, heart, and the kidneys. The goal of CytoSorb therapy is to reduce cytokine storm and the deadly inflammatory response through blood purification so that this injury may be mitigated or prevented. CytoSorb is plug-and-play compatible with the most commonly used blood purification machines or pumps in the intensive care unit used to treat COVID-19 patients, including hemoperfusion, hemodialysis, continuous renal replacement therapy (CRRT), and extracorporeal membrane oxygenation (ECMO) machines.
Mr. Vincent Capponi, Chief Operating Officer of CytoSorbents commented, “We greatly appreciate the FDA’s recognition, through this EUA, of the potential of CytoSorb and extracorporeal blood purification to help patients stricken with this terrible illness. It was clear in this truly collaborative process with the FDA, that the Agency was committed to urgently providing physicians and patients with new treatment options in the fight against COVID-19. We plan to continue working with the FDA to help as many patients as possible.”
See Full Press Release: U.S. FDA Grants CytoSorb® Emergency Use Authorization for Use in Patients with COVID-19 Infection
Written by: CytoSorbents Corporation

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.