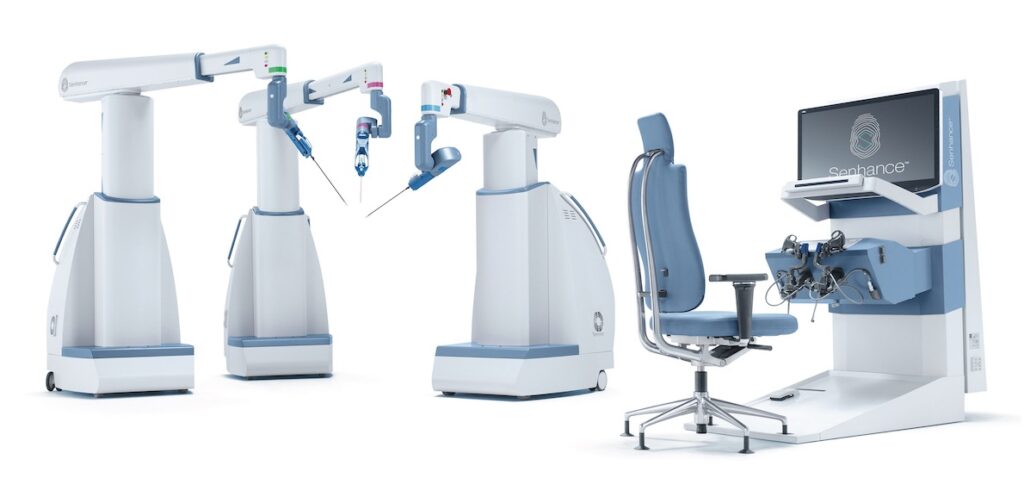
TransEnterix, Inc. (NYSE American: TRXC), a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery, today announced the Company filed a 510(k) submission with an Intelligent Surgical Unit (ISUTM)1 that is designed to enable machine vision capabilities on the SenhanceⓇ Surgical System.
“TransEnterix is the first company to seek FDA clearance for machine vision technology in abdominal robotic surgery. Rather than simply passing a video signal to the surgeon, the Intelligent Surgical Unit for Senhance will initially have the ability to actually visualize the surgical field to guide movement and capture information,” said Anthony Fernando, TransEnterix president and CEO. “This technology advance is an important first step towards enabling augmented intelligence and we believe it will support continued machine vision driven advances in surgery performed with the Senhance Digital Laparoscopy Platform.”
The initial features of the Intelligent Surgical Unit (ISU) are designed to increase control in visualization beyond what has previously been available in digital laparoscopy or robotic surgery. The cleared Senhance System already features unique eye-tracking camera control and the new technology would enable machine vision driven control of the camera for a surgeon by responding to commands and recognizing certain objects and locations in the surgical field. The ISU hardware is also designed to be compatible with planned future augmented intelligence features such as scene cognition and surgical image analytics that are expected to continue to drive meaningful innovations in digital laparoscopy with Senhance.
“Many of us that perform and teach surgery firmly believe that the care of patients will be transformed by augmented intelligence and machine vision capabilities in the future,” said Dr. Amit Trivedi, chair of surgery at Hackensack Meridian Health Pascack Valley Medical Center and a participant in the design and usability studies conducted in support of the 510(k) submission of the ISU. “Imagine if a computer could be the best assistant you’ve ever had in surgery by anticipating and moving a camera effortlessly to maximize control of the visual field. I’m very excited about this new addition to the Senhance System.”
This Intelligent Surgical Unit will be compatible with the global installed base of Senhance Surgical Systems, and will be compatible with third-party vision systems that are currently supported by Senhance.
See Full Press Release: TransEnterix Announces Submission of 510(k) to FDA for First Machine Vision System in Robotic Surgery | TransEnterix, Inc.
Written by: TransEnterix

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.