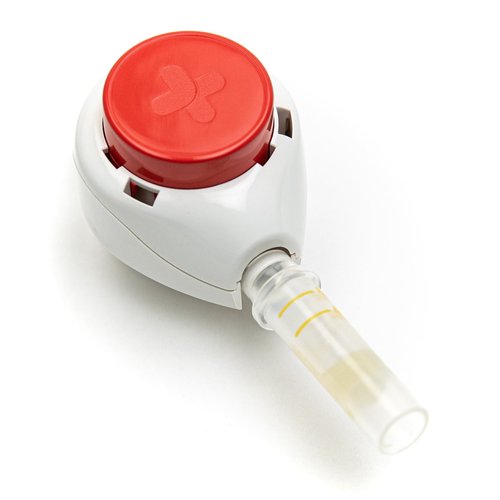
Tasso, Inc., the leading provider of convenient, clinical-grade blood collection solutions, announced that the U.S. Food and Drug Administration (FDA) has cleared its Tasso+™ lancet as a Class II medical device. The clearance allows the company to market and sell the device to more pharmaceutical companies, healthcare organizations, and academic institutions across the country, expanding access to a simple and virtually painless blood collection experience.
The Tasso+ device is the first single-use, patient-centric blood collection product to receive Class II clearance from the FDA as part of the new reclassification process for lancets, which are intended to puncture the skin to obtain drops of capillary blood samples. This action is part of the FDA’s ongoing effort to ensure the safe and effective use of blood lancets in the home and health care settings. With this clearance, more pharmaceutical companies will be able to accelerate their decentralized clinical trials, especially for pharmacokinetic and biomarker research endpoints. Healthcare systems and physicians will also be able to use the Tasso+ device with compatible collection tubes to make determinations on blood chemistries for their patients and expand care access. Tasso+ device-based solutions with Class II clearance will be widely available to customers across the country in Q4 2022.
“With continued industry interest in decentralized clinical trials and diverse testing applications, demand for our high-quality, virtually painless, convenient blood collection solutions is at an all-time high,” said Ben Casavant, PhD, CEO and co-founder of Tasso. “This FDA Class II medical device clearance will help improve patient care by relieving traditional phlebotomy-related bottlenecks and enabling more individuals to get the tests they need at the time they are needed. We are excited to unlock a new wave of large commercial opportunities for the company and to lead the industry into the future of remote testing.”
For more information on the Tasso+ device-based solutions with Class II clearance, please visit www.tassoinc.com/contact.
About Tasso
Trusted diagnostics made easy. Tasso is an emerging healthcare technology company that is transforming the traditional blood collection paradigm with a patient-centric approach. The company’s devices enable simple, convenient, and virtually painless blood collection for users. Tasso technology has the power to bring healthcare anywhere, any time. Headquartered in Seattle, Washington, Tasso is privately held and funded by federal grants, venture investments, and co-development agreements with industry leaders. For more information, please visit www.tassoinc.com.
See Full Press Release at the Source: Tasso+™ Device Earns FDA 510(k) Class II Medical Device Clearance
Press Release by: Tasso, Inc.
Legacy MedSearch has more than 35 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 17 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.