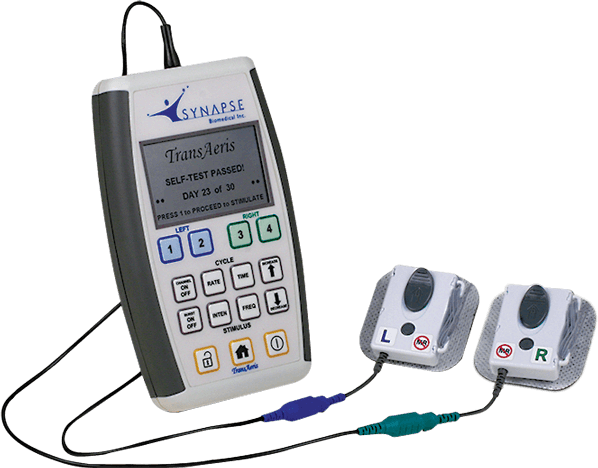
Synapse Biomedical, Inc. has received an Emergency Use Authorization for the emergency use of its TransAeris® DPS, to assist in weaning patients determined by their healthcare provider to be at high risk of weaning failure off of ventilators in healthcare settings during the COVID-19 pandemic for no more than 30 days.
During the COVID-19 pandemic, the surge of patients requiring prolonged mechanical ventilation (PMV) has put an unprecedented demand on hospital and ICU resources. Even when their primary symptoms have stabilized, these PMV patients are still at risk for developing ventilator-induced diaphragm dysfunction (VIDD)—further prolonging their ventilation.
The TransAeris system addresses this issue by conditioning a patient’s diaphragm to reduce or avoid VIDD. Models suggest the technology—which recently received CE Mark approval and is under clinical investigation in the U.S – could reduce ventilator burden in COVID-19 patients by 26 percent, helping to free up more ventilators in a time of great demand.
“More than 2,000 patients have been successfully treated world-wide with our diaphragm, pacing technologies,” said Anthony Ignagni, President & CEO of Synapse Biomedical. “We welcome the FDA’s leadership in providing this emergency pathway to get our latest TransAeris device into the hands of clinicians so they can help as many COVID-19 patients as possible during this pandemic.”
See Full Press Release: Synapse Biomedical, Inc. has received an Emergency Use Authorization for the emergency use of its TransAeris® DPS
Written by: Synapse Biomedical

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.