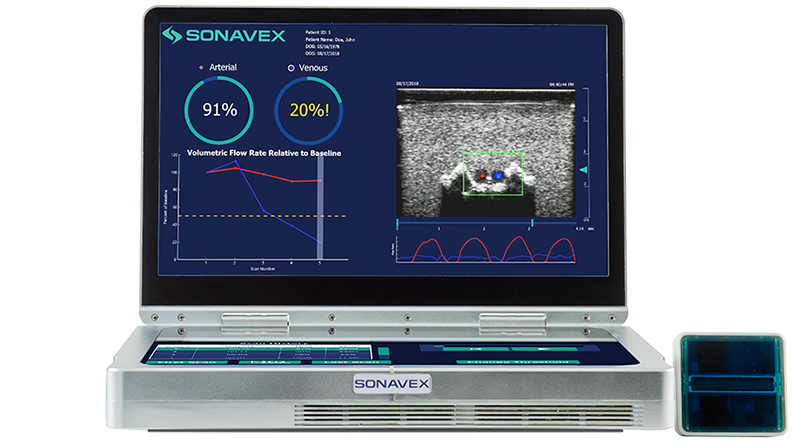
Following peripheral vascular and microvascular surgeries, it is important to be able to monitor how blood is flowing through the treated vessels and whether there may be an occlusion or compromise. This monitoring typically requires a nurse or a trained sonographer to use Doppler ultrasound, but now a new option is available.
Sonavex, a company based in Baltimore, won FDA clearance for its EchoSure system that combines 3D ultrasound with deep learning algorithms that can nearly completely automate the process of blood flow monitoring. The system provides both visual and quantitative outputs for quick and intuitive understanding of the situation.
The system uses Sonavex’s recently cleared EchoMark bioresorbable markers, which are bioresorbable polymeric implants, to calibrate to the imaging site and in the process making specialized sonography expertise unnecessary.

The system, once setup and monitoring the patient, can share its readings with the surgeon who performed the original procedure via an app. This can allow the physician to make decisions remotely as to what to do in case there’s a change in patient status.
“For decades, the surgical community has sought a simple, fast and non-invasive way to accurately quantify blood flow after microvascular and vascular surgeries,” said Devin O’Brien Coon, MD, Chief Medical Officer and President of Sonavex and a board-certified plastic and microvascular surgeon at Johns Hopkins. “Putting ultrasound technology in the hands of bedside nurses for the first time may enable detection of vascular compromise earlier than clinical observation alone, providing opportunities for more rapid intervention and improved patient outcomes.”
See Full Article at the Source: Sonavex EchoSure Vascular Blood Flow Monitor Cleared in U.S. | Medgadget
Written by MedGadget Editors
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.