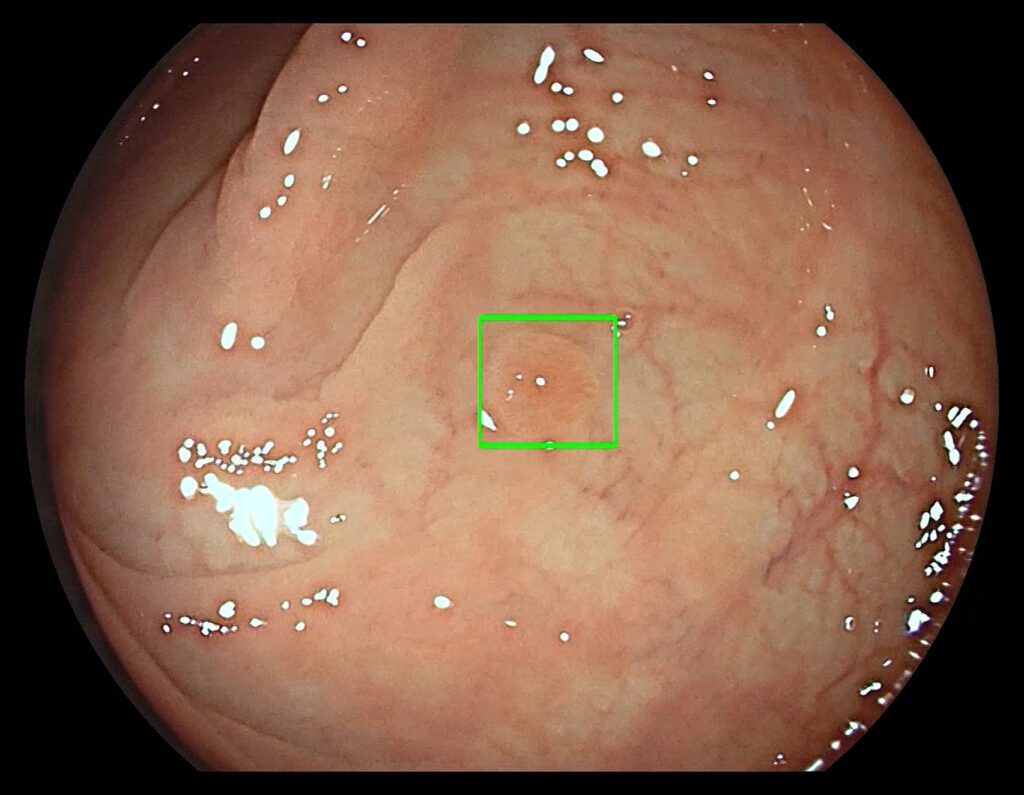
Medtronic plc, the global leader in medical technology, announced the launch of GI Genius™ intelligent endoscopy module at UEG Week in Barcelona, Spain. The GI Genius™ module is the first system worldwide using artificial intelligence to detect colorectal polyps. It provides physicians with a powerful new solution in the fight against colorectal cancer.
The GI Genius module uses advanced artificial intelligence to highlight the presence of pre-cancerous lesions with a visual marker in real-time – serving as an ever vigilant second observer. Studies have shown that having a second observer can increase polyp detection rates; every 1 percent increase in adenoma detection rate reduces the risk of colorectal cancer by 3 percent.1 Colorectal cancer (CRC) is the third most common form of cancer globally with 1.8 million new cases every year.2
“One of the key factors to maximizing the prevention of colorectal cancer is the integration of advanced technologies like artificial intelligence into daily practice. The use of artificial intelligence in gastroenterology ushers in a new era of diagnostic endoscopy that can improve the quality of colonoscopies. In my experience, the GI Genius module can be extremely precise in identifying lesions in the colonic mucosa that can be difficult to detect and may have been missed. This change in daily practice has the potential to improve diagnosis and overall outcomes for patients that may have colorectal cancer,” said Prof. Alessandro Repici, head of gastroenterology at Humanitas Hospital in Rozzano (Milan).
“Medtronic is focused on preventing colorectal cancer by early detection of pre-cancerous polyps with AI-assisted technologies,” said Giovanni Di Napoli, vice president and general manager of the Gastrointestinal & Hepatology business, which is part of the Minimally Invasive Therapies Group at Medtronic. “The GI Genius module automatically detects polyps, including small flat polyps that may go undetected thus increasing accuracy and reducing the risk of interval cancers which can occur between colonoscopies.”
To bring this technology to market, Medtronic entered a worldwide distribution agreement with Cosmo Pharmaceuticals. Cosmo Pharmaceuticals is the sole manufacturer of the artificial intelligent software and device. GI Genius intelligent endoscopy module is CE-marked and is available in select European markets. GI Genius intelligent endoscopy module does not have FDA clearance and therefore is not available for sale in the United States.
See Full Press Release: Medtronic Launches the First Artificial Intelligence System for Colonoscopy at United European Gastroenterology Week 2019 | Medtronic
Written by: Medtronic

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.