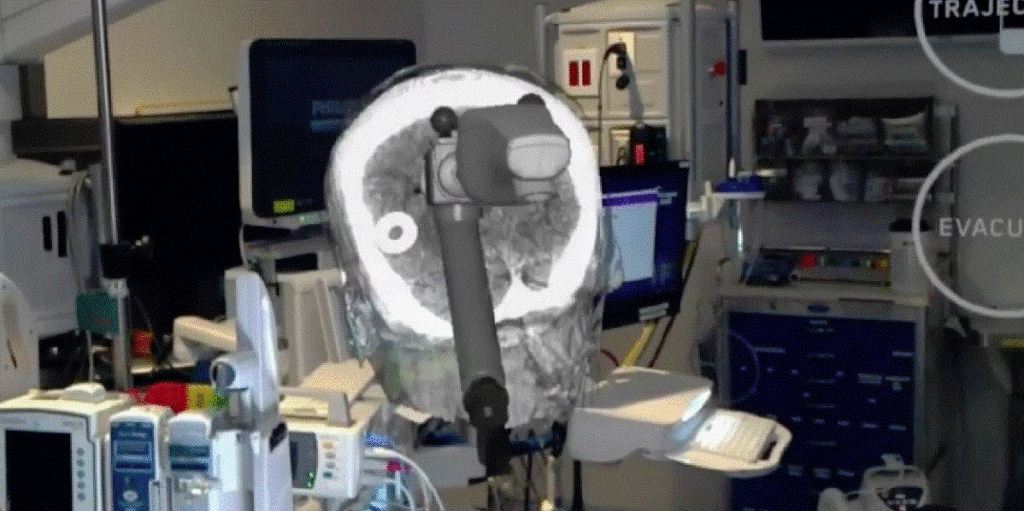
Medivis announced today that its groundbreaking augmented reality (AR) technology platform for surgical applications, SurgicalAR, has received 510(k) clearance for clinical use in the operating room by the U.S. Food and Drug Administration. The New York City based medical technology company will commence the immediate commercialization of the platform in the United States.
The enterprise SurgicalAR platform integrates the latest advancements in augmented reality, artificial intelligence, and computer vision to advance surgical visualization, thereby decreasing surgical complications and improving patient outcomes — all while lowering costs to the healthcare system.
“Holographic visualization is the final frontier of surgical imaging and navigation,” said Dr. Osamah Choudhry, neurosurgeon & CEO of Medivis. “The surgical world continues to primarily rely on two-dimensional imaging technology to understand and operate on incredibly complex patient pathology. ”
“Medivis introduces advancements in holographic visualization and navigation to fundamentally advance surgical intervention, and revolutionize how surgeons safely operate on their patients,” said Dr. Christopher Morley, radiologist & President of Medivis. “Achieving this milestone accelerates our mission to improve surgical precision and safety by allowing surgeons to see the invisible.”
This announcement comes on the heels of significant company momentum, including strategic partnerships with Verizon and Microsoft. Earlier this year, the company came out of stealth with $2.3M in funding, led by Initialized Capital. The company also recently released their AnatomyX platform for AR medical training.
“Medical imaging for invasive surgical procedures has been waiting for a transformation like this for decades —and we knew from day one that Medivis would be the leader in this emerging space,” Eric Woersching, General Partner, Initialized Capital.
See Full Press Release at the Source: Medivis Wins FDA Clearance for Breakthrough Augmented Reality Surgical System
Press Release by Medivis
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.