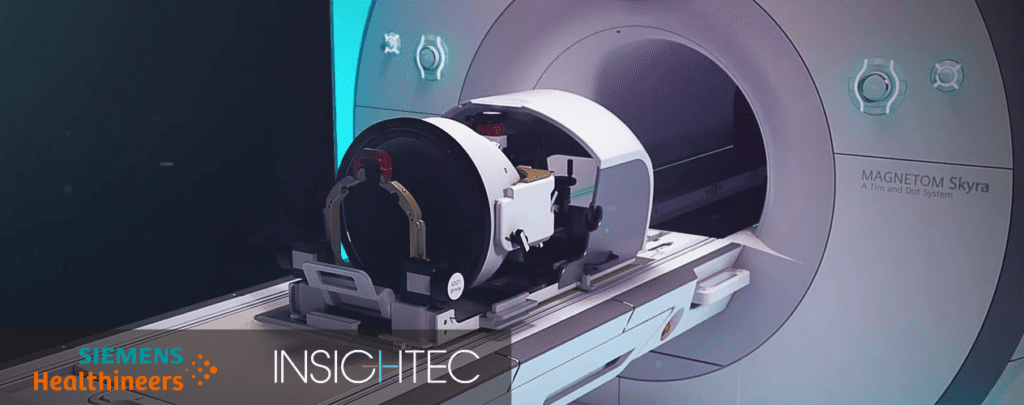
INSIGHTEC®, a global medical technology innovator of incisionless surgery, today announced that the U.S. Food and Drug Administration has approved Exablate Neuro™ compatibility for the state-of-the-art MRI Scanners Magnetom Skyra, Prisma and Prismafit from Siemens Healthineers to treat patients with essential tremor.
“This important milestone is directly attributed to the commitment and collaboration of the teams at INSIGHTEC and Siemens Healthineers, who met the challenge head-on,” said Maurice R. Ferré, MD, INSIGHTEC Chief Executive Officer and Chairman of the Board. “Expansion of MRI compatibility for Exablate Neuro substantially increases the potential reach of incisionless brain surgery for essential tremor patients.”
Exablate Neuro uses focused ultrasound to precisely target and accurately ablate tissue deep within the brain with no incisions. More than 1,500 medication-refractory essential tremor patients have been treated around the globe.
“With the compatibility of INSIGHTEC’s innovative technology with our MRI scanners, Siemens Healthineers is staying true to its mission of delivering best-in-class diagnostic imaging with advanced therapy solutions in the neurological space,” said Christoph Zindel, MD, Senior Vice President and General Manager Magnetic Resonance at Siemens Healthineers. “This joint development underlines our commitment to expanding precision medicine, and paving the way for offering focused ultrasound to more hospitals and patients in our worldwide network.”
“Focused ultrasound is an exciting technology, through which radiology and neurosurgery expand efforts together to advance best patient care,” said Satoshi Minoshima, M.D., Ph.D., Professor and Chair in the Department of Radiology and Imaging Sciences at the University of Utah, where the Exablate is currently being installed on a Magnetom Skyra from Siemens Healthineers. “The expansion of Exablate Neuro to be compatible with Siemens Healthineers MRIs will broaden access for patients to an incisionless treatment option.”
Exablate Neuro is FDA approved to treat medication-refractory essential tremor and clinical trials are ongoing to evaluate potential applications in the treatment of various neurological diseases.
See Full Press Release at the Source: INSIGHTEC Receives FDA Approval for Exablate Neuro Compatibility with Siemens Healthineers MRI Scanners
Press Release by INSIGHTEC & Siemens Healthineers
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.