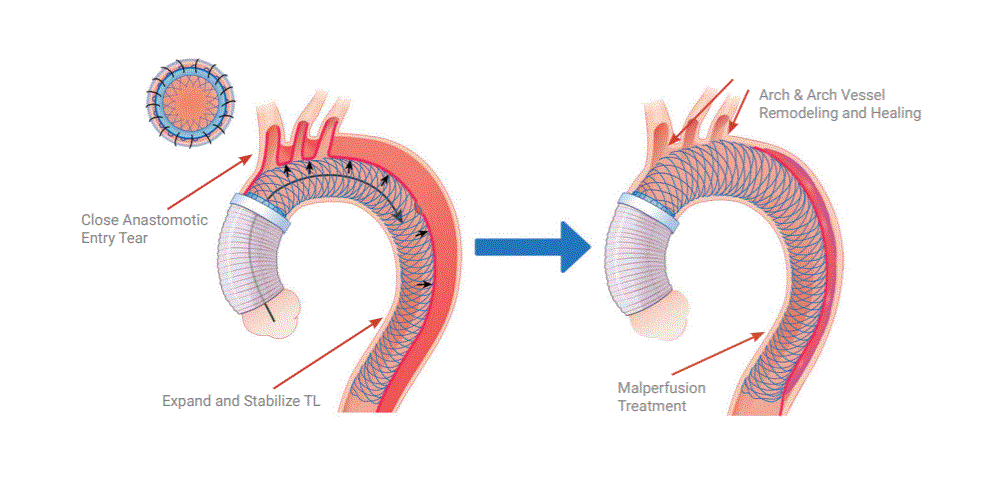
Ascyrus Medical announced yesterday that it has received Breakthrough Device Designation from the FDA for its Ascyrus Medical Dissection Stent (AMDS) to treat acute Type A aortic dissections. This designation serves as validation of the clinical importance of the AMDS and represents a significant milestone in the continued advancement of the technology globally. Ascyrus Medical conducted the largest prospective, monitored type A dissection trial (DARTS) with patients enrolled in Canada and Germany. The results have shown a significant reduction in mortality and re-interventions along with effective malperfusion management, without any device related adverse events.
The FDA’s Breakthrough Devices Program is intended to accelerate the regulatory review process for certain medical devices and device-led combination products that provide a more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. The goal of the program is to provide patients and health care providers with timely access to important breakthrough medical devices by accelerating their development, assessment, and review, while preserving the statutory standards for premarket approval, 510(k) clearance, and De Novo marketing authorization, consistent with the Agency’s mission to protect and promote public health.
An acute type A dissection is a life-threatening condition that requires emergent repair. Today’s standard surgical treatment is associated with a high rate of mortality and reintervention rates due to malperfusion and continued growth of the aorta. The AMDS complements the current surgical repair without adding significant time or complexity to the procedure. It’s designed to reduce the risk of mortality and reoperations by treating malperfusion and inducing positive aortic remodeling.
Dr. Ali Shahriari, CEO of Ascyrus Medical, said, “Receiving Breakthrough Device Designation will accelerate our efforts and partnership with the FDA to secure a US approval for the AMDS. This is aligned with our commitment to offer life-saving and innovative products, supported by rigorous clinical data, to make a difference in patient’s lives. We look forward to close collaboration with the FDA and our clinician partners to continue to advance the treatment options for patients suffering from this deadly disease.”
See Full Press Release at the Source: FDA Grants Breakthrough Device Designation to Ascyrus Medical Dissection Stent (AMDS)
Press Release by Ascyrus Medical
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device IndustryLegacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.