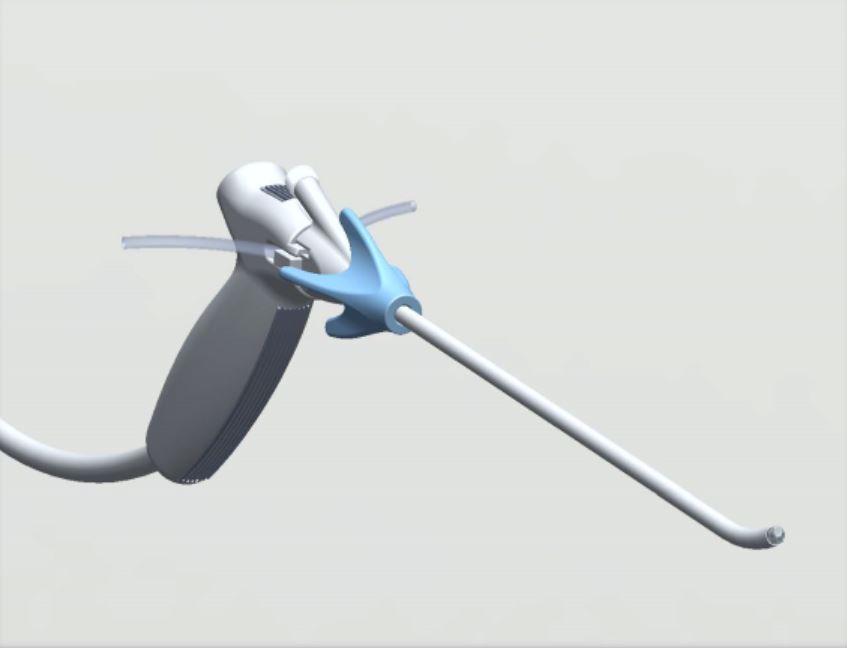
Luminelle DTx Hysteroscopy System has received its 510(k) clearance from the FDA with a dual-indication for both hysteroscopy and cystoscopy. The Luminelle DTx Hysteroscopy System is small, easy to use, cost effective and has the potential for an affordable, accurate patient diagnosis and/or therapeutic procedure in one doctor’s office visit. The system went from concept to commercialization in just 25 months time and was designed and produced using 95% United States based supply chain.
Today, about 76 percent of hysteroscopy procedures take place in the operating room.
“Since the best technology is typically only available in the operating room, many physicians and patients opt to skip the hysteroscopy and either perform a blind biopsy or go straight to surgery, without taking a good look inside of the uterus,” explains Dr. Amy Garcia, MD FACOG; Director – Center for Women’s Surgery; Institute for Hysteroscopy Training, Albuquerque, NM. “An in-office system that is easy to use, prevents a blind biopsy and allows the physician to offer either a therapeutic diagnosis or a biopsy under visualization elevates the standard for uterine care.”
The technology of the Luminelle DTx Hysteroscopy System allows physicians to see the uterus’ internal lining from an optimal distance with clarity thus enabling identification of suspicious tissue, perform a biopsy under visualization, and offer a potentially faster diagnosis, treatment and recovery time for the patient.
“Most operating room equipment is cost and space prohibitive for standard office use. We created an elegant and ergonomic system with visualization quality comparable to that of the OR, and that is comfortable for both physician and patient,” explains Allison London Brown, CEO of UVision360, the medical device company responsible for the rapid design and launch of the device. “Our system is a fully-integrated hysteroscopy/cystoscopy system with a value-based cost to provide better accessibility for the changing needs of today’s GYN. The combined features lead to the potential for better care for women, earlier detection, and improved health outcomes.”
Read More at the Source: FDA Approves New FemTech Device to Enhance Quality of Care for the Uterus
Press Release by UVision360
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
