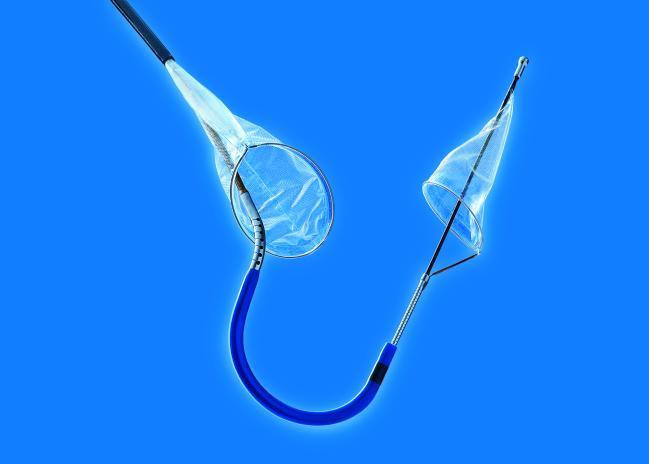
The US Food and Drug Administration (FDA) has cleared the Sentinel Cerebral Protection System (Claret Medical), a filter device used during transcatheter aortic valve replacement procedures to reduce the risk of stroke caused by embolic debris, the company announced today.
The clearance comes after a mostly positive review by the agency’s advisory committee back in February—although that committee took no formal vote. As reported by TCTMD, most panelists agreed that potential benefits of protection using the device outweigh any risks and said that despite the lack of clear clinical benefit —the SENTINEL trial did not meet its primary efficacy endpoint of significant reductions in new brain lesion volume on MRI—they themselves would want to have the device in place if they were undergoing TAVR.
“Intuitively, it seems like a very good thing to me that these [bits of] debris are taken out of the circulation pretty effectively by this filter and I think that’s a good thing,” FDA advisory panel member Jeffrey S. Borer, MD (SUNY Downstate Medical Center, New York, NY), said during the Sentinel’s review. “Even though I really can’t interpret what it means, I intuitively believe that the images that were done look better when a filter was used than when a filter wasn’t used. . . . I’m troubled that I don’t have a clear position that I can take. However, if I had to, if I was being forced to [choose], I’d rather have this thing in than not have it in, if I had to have a TAVR.”
The 363-patient SENTINEL trial was conducted at 18 US and European centers and led by Susheel Kodali (NewYork-Presbyterian Hospital/Columbia University Medical Center New York, NY), and Samir Kapadia, MD (Cleveland Clinic, OH). In the trial, patients undergoing TAVR were randomized 2:1 to cerebral protection or no cerebral protection, with further randomization to safety follow-up or MRI and neurocognitive follow-up groups. At 30 days, MACCE rates in the cerebral-protection group were noninferior to the performance goal and not statistically different from those of patients treated without the protection device (7.3% vs 9.9%; P = 0.41). In the MRI group, new lesion volume was numerically lower but not statistically different between the protection and no-protection groups (102.8 mm3 vs 178.0 mm3, P = 0.33). However, after adjusting for baseline lesion volume and valve type, there was a significant reduction in new lesion volume in protected territories (P = 0.02) with embolic protection. Of note, debris was captured in 99% of cases.
Read More from the Source: FDA Clears Sentinel Cerebral Protection Device for Use During TAVR | tctmd.com
By Shelley Wood
Photo Credit: Claret Medical