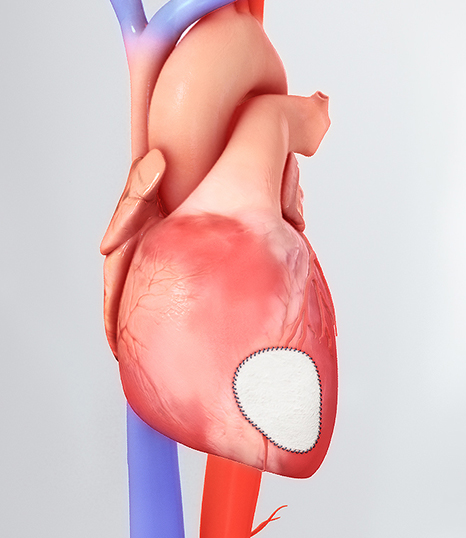
CorMatrix® Cardiovascular, Inc., a leading developer of regenerative cardiovascular medical devices, today announced FDA 510(k) clearance for the Cor™ PATCH. The Cor™ PATCH is indicated for epicardial tissue support and repair in adult patients. The Cor™ PATCH epicardial patch is the first epicardial cardiovascular medical device composed of first next generation CorMatrix® ECM® to be cleared by the FDA and introduced into the US cardiac surgery market. The applications and clinical description of uses include the epicardial support and repair of atria and/or the right and left ventricular walls of the heart that have been thinned or damaged as a result of myocardial infarct. The Cor™ PATCH is first in a series of products to address clinical challenges in the cardiovascular disease market, to include regenerative and innovative design solutions for congestive heart failure, and adult or pediatric cardiac valve patients.
“We have a unique opportunity to enhance the repair and recovery of heart muscle after injury using the Cor™ PATCH technology. This could be a game changer for patients undergoing surgical procedures aimed to increase the blood supply to damaged areas of their heart. My translational research provides an important foundation of data to support the clinical use of this technology as an epicardial patch during coronary bypass surgery. We can now directly target damaged muscle in addition to bypassing blocked vessels,” said Dr. Paul W.M. Fedak, Professor of Cardiac Surgery, Cumming School of Medicine, University of Calgary, Libin Cardiovascular Institute of Alberta.
“The ECM® technology and our knowledge of the underlying physiological mechanisms for regeneration continues to rapidly grow. The Cor™ PATCH represents our entry into epicardial tissue repair and support. Our pipeline is becoming full of some very compelling products,” said Robert G Matheny, MD Chief Medical & Scientific Officer, CorMatrix®Cardiovascular Inc.
“The Cor™ PATCH is the first next generation ECM® product cleared by the FDA for epicardial tissue support and repair. The introduction of the Cor™ PATCH epicardial patch to the cardiac surgery market represents the first of a rapidly developing CorMatrix® pipeline of next generation ECM® products and patient solutions,” said Edgar Rey, President & CEO, CorMatrix® Cardiovascular Inc.
See Full Press Release at the Source: CorMatrix® Cardiovascular, Inc. receives FDA 510(k) clearance to market the Cor™ PATCH epicardial patch for tissue support and repair in adult patients
Press Release by CorMatrix
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.