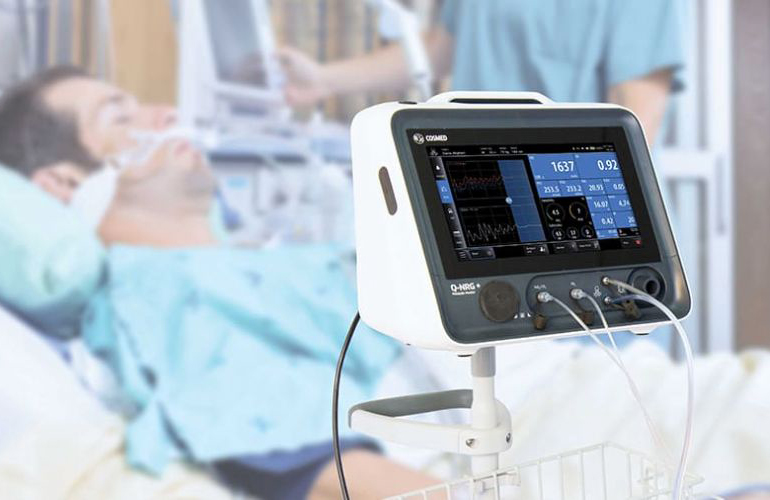
Baxter International Inc. (NYSE: BAX), a global leader in clinical nutrition, today announced the U.S. Food and Drug Administration (FDA) clearance of Q-NRG+, a metabolic monitoring device utilizing indirect calorimetry (IC) technology. IC is considered the “gold standard”1 to accurately measure a patient’s calorie needs, or resting energy expenditure (REE).
These readings can help inform prescription and administration of nutrition therapy, which may include parenteral nutrition (PN), the intravenous administration of nutrients. Q-NRG+ is expected to be available in the United States beginning at the ASPEN 2020 Nutrition Science & Practice Conference taking place March 28 – 31, 2020 in Tampa, Florida.
As part of its partnership with COSMED, Baxter has rights to bring Q-NRG+ to at least 18 markets around the globe, with the potential for further expansion. The 510(k) clearance is the latest regulatory approval for Q-NRG+, which is currently available in 12 countries in Europe as well as Canada, Australia and New Zealand.
Hospital malnutrition is a serious public health issue in the United States. Approximately two million hospital stays in the U.S. involve malnutrition,2 and almost a quarter of U.S. patients with malnutrition were readmitted to the hospital within 30 days of discharge.3 Hospitalized patients with malnutrition may require PN therapy if they cannot be fed orally or enterally (tube feeding).
Traditional methods of estimating caloric requirements use predictive equations that are based on factors like weight, height, gender and age. However, research has shown that these equations often result in inaccurate measurements and could result in overfeeding or underfeeding.4, 5
“It can be challenging to prescribe clinical nutrition without knowing the exact caloric needs of a patient,” said Heather Knight, general manager, U.S. Hospital Products, Baxter. “With Q-NRG+, clinicians will have access to the latest technology to accurately measure energy requirements and won’t have to rely on predictive equations or dated technology — which can be cumbersome and time consuming.”
See Full Press Release: Baxter and COSMED Announce U.S. FDA 510(k) Clearance of Q-NRG+ Indirect Calorimetry Device | Baxter
Written by: Baxter and COSMED

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.