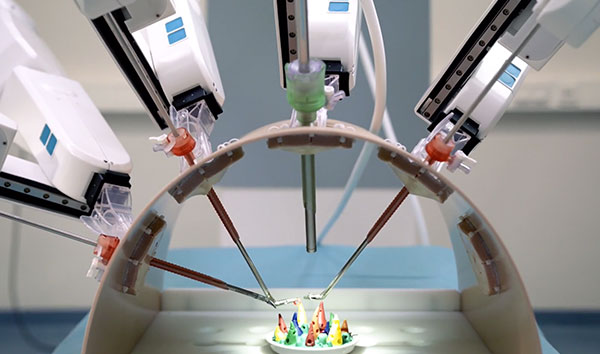
Avateramedical GmbH, an innovative German medical technology company, today announced the successful completion of the CE conformity assessment procedure for its avatera® system[1] for robot-assisted, minimally invasive surgery. All four components of the system – the surgical robot and control unit, the instruments, the endoscopes and the sterile components – can now carry the CE mark. This means that avatera is now approved in the European Economic Area for minimally invasive laparoscopy (“key hole surgery”), to this point primarily used in gynecology and urology.
The avatera system was developed based on current standards in robot-assisted surgery and optimized in close cooperation with future users – including surgeons and surgical teams – in terms of cost, quality, comfort and reliability. avatera’s unique single-use concept for instruments and sterile components guarantees functional, reliable instruments for every surgical procedure, reduces the risk of contamination and associated patient infections, and avoids the cleaning and sterilization necessary with reusable instruments. Compared to classical laparoscopy, the success of robot-assisted surgery is based on the high level of comfort, precision and dexterity of the instruments. With its open design and low noise level, the avatera system also enables smooth communication in the operating room.
“The successful completion of the CE conformity assessment is an important milestone in our young history,” commented Dr. Hubertus von Gruenberg, co-founder and CEO of the avateramedical group. “We are very proud to offer our ‘German Robot’, which is developed and produced in the two Thuringian sites of the Company – Jena and Ilmenau – as a competitive solution in this strongly growing market. In the next step, we will introduce the avatera system to clinical practice.” At the same time, avateramedical’s management announced its strategic plan to build one of the most modern and largest production facilities for medical robotics in Germany in 2020 to support increased demand anticipated over the next five years. “We are confident in our plan to launch avatera in 2020, aspiring to significantly improve the uptake and access to robot-assisted surgery in Europe,” concluded Dr. von Gruenberg.
The global market for surgical robots currently stands at USD 4.5 billion with experts estimating growth to USD 13 billion by 2025.[2] Current statistics underpin this growth potential with about 8.5 robotic systems (also called telemanipulator systems) available per 1 million inhabitants in the U.S. compared to only 1.0 surgical robots per 1 million inhabitants in Europe. With an ageing population, increasing demands on hospital efficiency and the growing need of surgeons and patients for better technologies, robotic surgery could soon become the gold standard in European hospitals.
Avateramedical’s launch plans are financially fully supported by Tennor Holding B.V, its seed investor and majority shareholder. Tennor recently granted a new convertible loan facility to avateramedical and is committed to supporting the international product roll-out and to financing the construction of a state-of-the-art manufacturing plant for the Company in Germany. Lars Windhorst, Chairman of the Advisory Board of Tennor Holding B.V., said: “Since avateramedical’s foundation in 2011, the successful development of the Company has confirmed our early investment decision and our trust in avateramedical’s capability to leverage the enormous market potential for robot-assisted surgery. The current indication of interest from a broad base of European and international prospective customers, combined with the Company’s imminent product launch across Germany and Europe, give us confidence that avateramedical can become an important global player in this strongly growing market.”
Avateramedical also substantially strengthened its corporate governance by proudly announcing the appointment of prominent candidates in the areas of medical device manufacturing, information technologies, artificial intelligence and surgery to the Supervisory Board of avateramedical. Joe Hogan will serve as Chairman of the Supervisory Board. Mr. Hogan is currently President and CEO of Align Technology, a c. USD 2.2 billion revenue global medical technology leader in the clear aligner market. Previously, he was CEO of ABB Ltd. and CEO of GE Healthcare. Arvind Sodhani, founder and former CEO of Intel Capital, Santa Clara (USA) will be also member of the Supervisory Board. Intel Capital is one of the world’s largest global technology investors and has invested north of USD 12.3 billion in more than 1500 companies since 1991. As a third member, Dr. Jay Austen, Chief of the Division of Plastic and Reconstructive Surgery at the Massachusetts General Hospital and Professor of Surgery at Harvard Medical School, will join the Supervisory Board of avateramedical.
See Full Press Release: CE Mark for avatera®, the First German System for Robot-Assisted, Minimally Invasive Surgery, Setting the Foundation for Strategic Growth Plans –
Written by: Avatera

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.