Allurion Technologies, an established leader in the development of innovative, proven, and trusted weight loss experiences, today announced the Premarket Approval (PMA) submission of its flagship Elipse gastric balloon. The company had previously announced the successful completion of its landmark FDA pivotal study and the hiring of key personnel who will be leading the US market launch. These milestones lay the foundation for the company’s eventual entry into the US market.
Allurion also announced that Joyce Johnson, a medical device industry executive with over 25 years of compliance experience, has joined the company as Vice President, Regulatory Affairs and Quality Assurance.
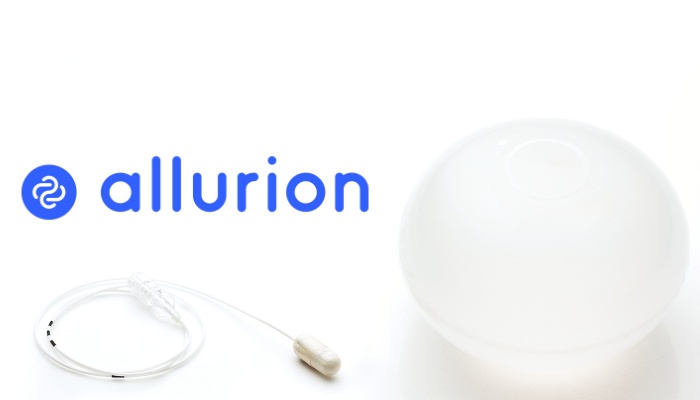
Allurion’s weight loss program features the Elipse Balloon—the world’s first and only procedureless medical device for weight loss. Unlike other weight loss balloons on the market, the Elipse Balloon is designed to be placed and removed without surgery, endoscopy, or anesthesia. It is swallowed in a capsule during an outpatient office visit and is designed to remain in the stomach for approximately four months, after which it opens and passes naturally from the body.
An estimated 160 million Americans are either obese or overweight. Nearly three-quarters of American men and more than 60% of women are obese or overweight.i According to a recent study published in the New England Journal of Medicine, rates of overweight and obesity will continue to rise precipitously over the next ten years. By 2030, 85% of Americans will be overweight and 100 million will be obese.ii Overweight is defined as having a Body Mass Index (BMI) greater than or equal to 25 and lower than 30, while obesity is defined as having a BMI equal to or greater than 30.
Allurion has already treated over 20,000 patients in over 30 countries across the world outside the US. In one of the largest balloon weight loss studies ever conducted overseas, the Elipse Program resulted in nearly 30 pounds of weight loss on average across 1,623 patients. The study was named a Top 10 Abstract at IFSO 2019.
Executive Vice President of Global Commercial Benoit Chardon added, “Allurion is looking forward to entering the US market where the burden of obesity continues to grow. The investments we have made in expanding our global business and preparing for US launch underscore our commitment to tackling the obesity epidemic all around the world.”
As the company prepares for entrance into the US market in late 2020, it has filled a key executive position in regulatory affairs and quality assurance with the addition of Joyce Johnson as Vice President. At Allurion, Ms. Johnson will oversee all global compliance and product submission activities as the company expands its offerings and enters new global markets. She will be the company’s primary regulatory contact with the FDA for ongoing approval discussions and assume responsibilities with existing global regulatory bodies. Ms. Johnson comes to Allurion with over 25 years of experience in the medical device industry where she has held key positions in global compliance and product registration. Most recently, she held the position of Vice President, Regulatory and Quality at SpaceLabs. Prior to SpaceLabs, Ms. Johnson served in several Vice President capacities at Smith & Nephew, Analogic and Draeger.
“Joyce comes to Allurion at an opportune and exciting time as we work towards receiving US regulatory approval from the FDA for the Elipse Balloon,” said Shantanu Gaur, M.D., co-founder and Chief Executive Officer of Allurion. “Her track record of success and leadership in the areas of compliance and quality assurance will prove valuable as we continue to rapidly scale the business.”
See Full Press Release: Allurion Technologies Announces Submission of US Premarket Approval (PMA) Application for its Flagship Elipse® Gastric Balloon | Business Wire
Written by: Allurion Technologies

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.