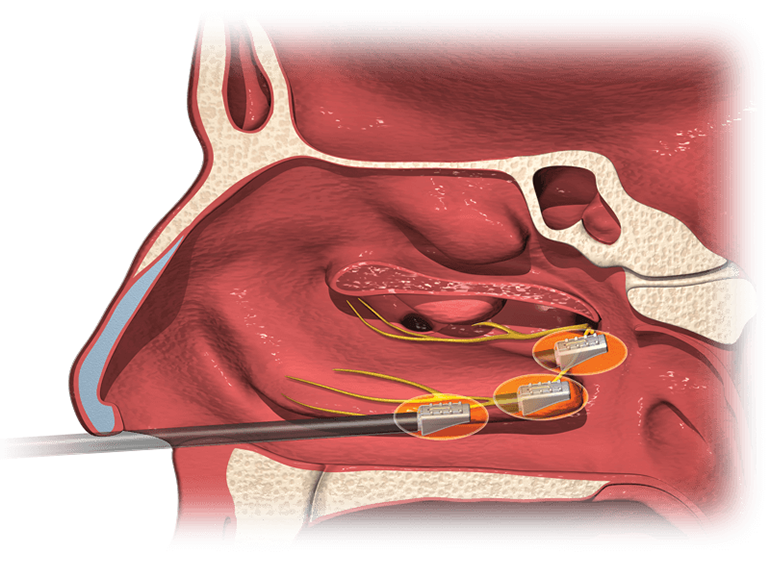
Aerin Medical Inc., a company focused on minimally invasive solutions for chronic nasal conditions, today announced U.S. Food and Drug Administration (FDA) clearance and U.S. launch of the company’s second product, the RhinAer™ Stylus, an innovative device for nonsurgical treatment of chronic rhinitis.
More than 30 million Americans suffer from nonallergic rhinitis.1 Patients with the condition suffer from runny nose, post-nasal drip, congestion, chronic cough, and other symptoms. For many patients, management with medications and sprays is inadequate. The RhinAer procedure provides lasting relief by directly disrupting the signals that cause symptoms. The procedure can be performed under a local anesthetic in an ENT physician’s office, with no incisions and minimal discomfort.
“Chronic rhinitis can significantly affect quality of life and impact daily activities,” said Adil A. Fatakia, M.D., a rhinologist at West Jefferson Medical Center in Marrerro, La. “The RhinAer procedure’s compelling clinical results, including its high responder rate, clean side effect profile and tolerability, make it a highly attractive option for my patients.”
Clinical results from the prospective, multi-center pivotal clinical trial of the RhinAer Stylus demonstrated meaningful benefits to patients. In the study, 96% of patients treated with the RhinAer procedure reported an improvement in their rhinitis symptoms at six months, with symptoms improving on average by 61%. The procedure was safe and generally well-tolerated. Notably, significant improvements were demonstrated for runny nose and post-nasal drip, the most bothersome chronic rhinitis symptoms.
“The FDA clearance and launch of our second nonsurgical innovation for ENT physicians and patients is a significant milestone for Aerin,” said Fred Dinger, President and CEO. “More than 13,000 patients with nasal airway obstruction have now been treated with our first product, the VivAer® Stylus with the Aerin™ Console. With the addition of the RhinAer Stylus we now offer ENT physicians a platform solution that can be used to improve the lives of the millions of patients suffering from chronic rhinitis.”
See Full Press Release: Aerin Medical Announces FDA Clearance and U.S. Launch of Innovative Nonsurgical Procedure for Chronic Rhinitis | Business Wire
Written by: Aerin Medical

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.