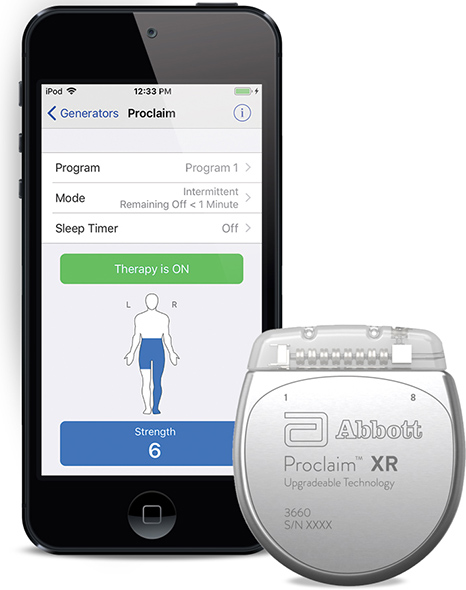
Abbott announced the U.S. Food and Drug Administration (FDA) has approved the company’s Proclaim XR™ recharge-free neurostimulation system for people living with chronic pain. The Proclaim XR platform offers a low dose of Abbott’s proprietary BurstDR™ stimulation waveform, which was created based on scientific insights from doctors and research to mimic natural patterns found in the brain. It works by using low doses of mild electrical pulses to change pain signals as they travel from the spinal cord to the brain. The delivery of lower doses of spinal cord stimulation (SCS) helps extend the system’s battery life, allowing people to experience pain relief, without the hassle of recharging, for up to 10 years*.
Abbott’s low-energy stimulation is based on finding the lowest effective dose as determined by the treating clinician for select patients. It delivers BurstDR stimulation intermittently, at low energy levels, while allowing people to experience the same level of superior pain relief. Proclaim XR also uses familiar Apple mobile digital devices and Bluetooth® wireless technology to help discreetly manage pain and fit seamlessly into a patient’s lifestyle.
The system was developed based on positive results from Abbott’s BurstDR micrOdosing stimuLation in De-novo patients (BOLD) study, which showed 100% of 24 enrolled patients on a low-energy BurstDR dosing program experienced pain relief with less than six hours of battery use per day, while approximately 50% of those patients achieved pain relief with the lowest effective dose (less than two hours of battery use per day). Even at the highest settings, the systems were still in use only 25% of the time. Data from this trial was presented at multiple society meetings including the North American Neuromodulation Society (NANS) and the International Neuromodulation Society (INS) earlier this year.
“For the 50 million people living with chronic pain in the United States this is a new and exciting treatment that is supported with evidence validated by the BOLD study, an established protocol for titrated intermittent dosing to give patients individualized pain relief while using therapy for 6 hours or less per day,” said Timothy Deer, M.D., DABPM, president and chief executive officer of The Spine and Nerve Center of the Virginias in Charleston, W.Va. “Proclaim XR is a major advancement in spinal cord stimulation, and is an evidence-based therapy that is mobile app-based and features upgradeable software. This means patients won’t need surgery to benefit from future advances in this technology.”
Alternative stimulation systems may use higher frequencies or multiple waveforms that use more energy, requiring them to be recharged daily – taking time from a person’s day. Abbott’s Proclaim XR system allows physicians to identify the lowest effective dose of BurstDR stimulation customized to each patient, optimizing system longevity while maintaining effective pain relief, and eliminating the need for recharging (for up to 10 years at low-dose settings*). This patient-centric innovation is possible because of Abbott’s proprietary waveform and advanced battery technology that is integrated into Proclaim XR.
“Proclaim XR is a new and differentiated advancement in how we approach chronic pain management, offering a safe and effective non-opioid technology for up to a decade of pain relief,” said Jacqueline Weisbein, D.O., Napa Valley Orthopaedic Medical Group in Napa, Calif. “Having access to reliable pain relief – from a system that doesn’t have the hassle of needing to be recharged –is a gamechanger in helping people live their best lives, free from pain.”
See Full Press Release: FDA Approves Abbott’s “Low Dose,” Recharge-Free Spinal Cord Stimulation System with up to Ten Year Battery Life* for People Living with Chronic Pain – Sep 26, 2019
Written by: Abbott

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.