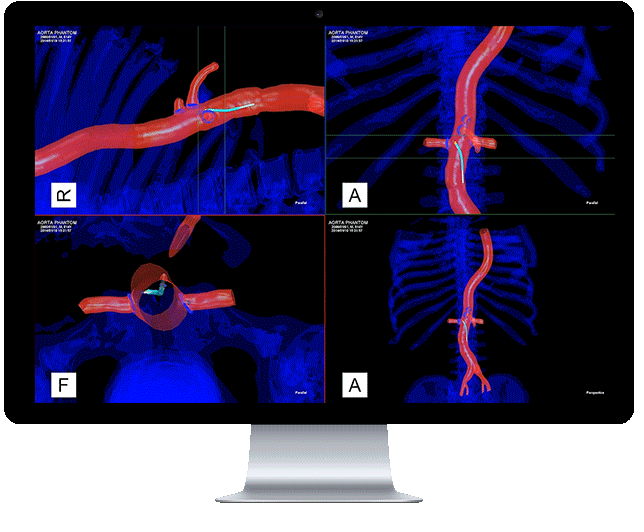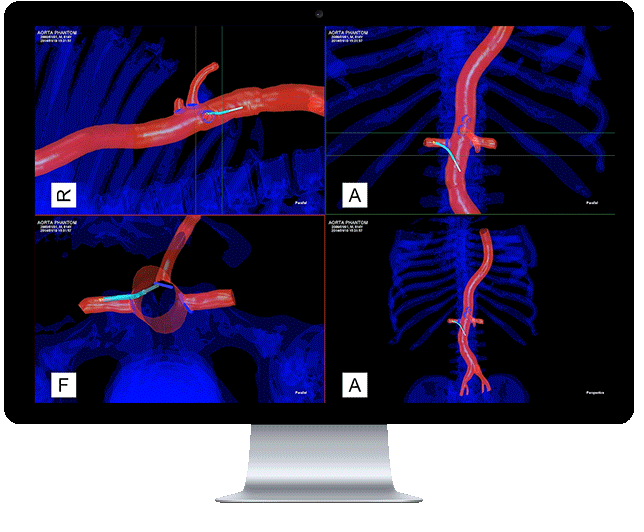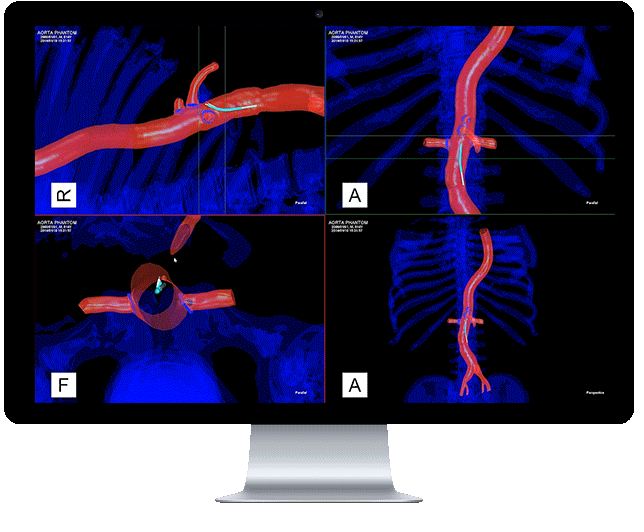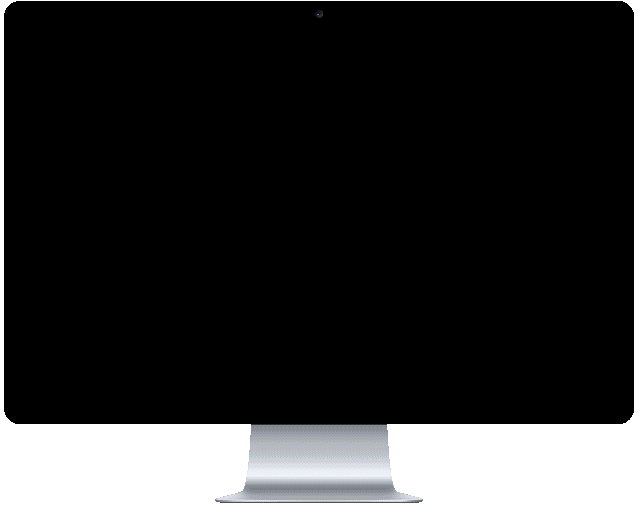
Centerline Biomedical, Inc. (Centerline) has announced that the company has received 510(k) clearance from the United States Food and Drug Administration (FDA) to market its flagship product, the Intra-Operative Positioning System, IOPS.
A non-radiation-based surgical navigation system for minimally invasive surgery, IOPS leverages anatomical mapping algorithms and electromagnetic tracking technology to provide three-dimensional color visualization and guidance in real time during endovascular procedures. The system, developed at Cleveland Clinic’s Heart and Vascular Institute, addresses the need for a path away from X-ray fluoroscopy which is currently the standard of care, despite severe visualization challenges.
“To date, Cleveland Clinic has partnered with some 87 companies; among these, Centerline has been particularly rewarding and we are very excited about them having received 510(k) clearance. Most certainly as partners, shareholders, and prospective customers, we at Cleveland Clinic look forward to continuing support of the exciting research and commercialization efforts of this rapidly growing company,” stated Jim Zalar, General Manager of Business Solutions at Cleveland Clinic Innovations and Cleveland Clinic’s appointee to the company’s Board of Directors.
Once in the market, Centerline hopes to show significant value to the technology by reducing procedure cost while improving radiation safety and patient outcomes. “Having worked closely with Centerline for almost five years, I am very pleased to see that IOPS will soon be commercially available,” remarked Dr. Matthew Eagleton, Chief of Vascular Surgery at Massachusetts General Hospital and Chair of the company’s Scientific Advisory Board. “We hope to use this game-changing technology to revolutionize our approach to vascular procedures and imaging.”
This milestone, achieved working with regulatory partner JALEX Medical, will allow marketing and commercial use of IOPS for endovascular interventions in the descending aorta, though the underlying platform will be applicable to many other minimally invasive procedures in the future. Such future indications may include structural heart interventions, an application the company is currently exploring with funding from an NIH small business grant. The company’s R&D efforts will continue in parallel with Centerline’s upcoming market launch.
See Full Press Release at the Source: Centerline Biomedical Receives FDA 510(k) Clearance for Intra-Operative Positioning System
Press Release by Centerline Biomedical
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.