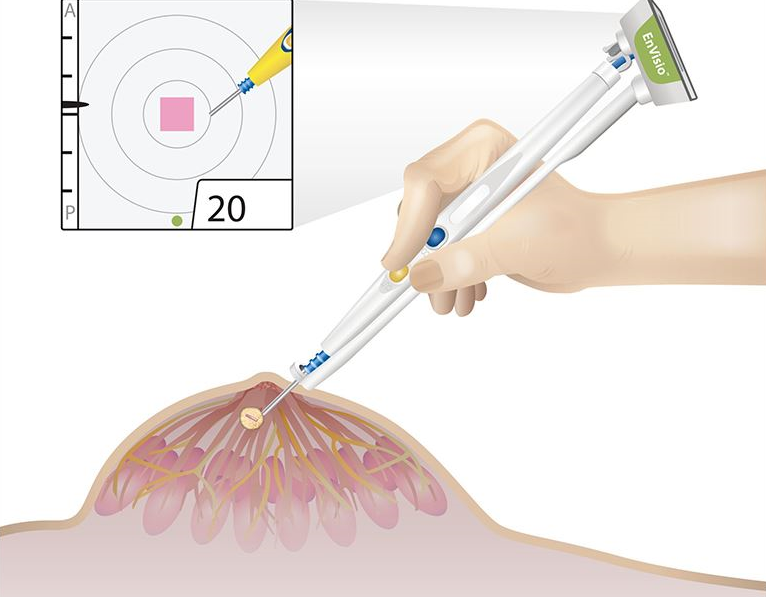
Elucent Medical, a company dedicated to developing better pathways for breast cancer care, today announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the clinical use of the company’s EnVisio™ Surgical Navigation System.
Placed at the time of the breast biopsy, Elucent Medical’s permanently implantable wireless SmartClip™ Soft Tissue Marker provides an alternative to painful and costly localization procedures, in which the standard approach is to place a hook-wire in the breast to identify the location of the tissue to be removed. The SmartClip™ is used by surgeons with the company’s EnVisio™ Navigation System to identify the location of the malignancy and to wirelessly navigate to it in real-time 3-D. The system can uniquely identify three different SmartClip™ Soft Tissue Markers – allowing physicians to effectively mark difficult lesions and navigate distances, depths, and directions – removing any clinical need for hook-wire solutions.
Elucent Medical was co-founded by Lee G. Wilke, M.D., Professor of Surgery and Director of the University of Wisconsin-Madison Health Breast Center. The company partnered with several leading breast surgeons across the country during the design and development of its solution.
“Elucent Medical is offering a cost-effective solution that addresses a key challenge for breast surgeons: how to easily find the location of a malignant biopsy during surgery, especially in tissue that lacks anatomical landmarks,” said Dr. Wilke. “We developed a system designed by surgeons to be intuitive, efficient, and precise, eliminating the need for a localization procedure. We believe this innovation has the potential to improve cosmetic and clinical outcomes, with other possible applications for lymph and thoracic surgeries.”
“Our vision is to disrupt the standard of care for women who receive localization prior to their lumpectomy as part of their breast cancer treatment,” said co-founder and CEO Laura King. “Today we take a significant step toward achieving this goal. The EnVisio™ Navigation System and SmartClip™ Soft Tissue Markers will provide patients and surgeons with a wire-free, minimally invasive alternative for pre-surgical planning and navigation throughout surgery.”
See Full Press Release at the Source: Elucent Medical Receives FDA Clearance for Innovation for Breast Tumor Removal Procedures | Business Wire
Press Release by Elucent Medical
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.