XACT Robotics, a pioneer in robotic navigation and steering solutions for percutaneous image-guided procedures, announced today that it has received CE Mark approval for its robotics navigation and steering system for image-guided percutaneous procedures, such as ablations and biopsies.
The Company also announced that it has raised $5 million in round C investment. The funds will mainly be used for the launch and operation of seven Centers of Excellence in the US, Europe and Israel, where the Company expects that hundreds of clinical procedures will be conducted within the next 12 months with the XACT robotic navigation and
steering system. The first Center of Excellence has already been launched earlier this year at the Hadassah Medical Center in Jerusalem, Israel, where the first cases were successfully completed as part of a clinical trial by Professor Nahum Goldberg, M.D., the Head of Interventional Oncology Unit and Director of the Applied Radiology Research Lab at Hadassah Medical Center, and the principal investigator in this study using XACT’s novel system to target lesions.
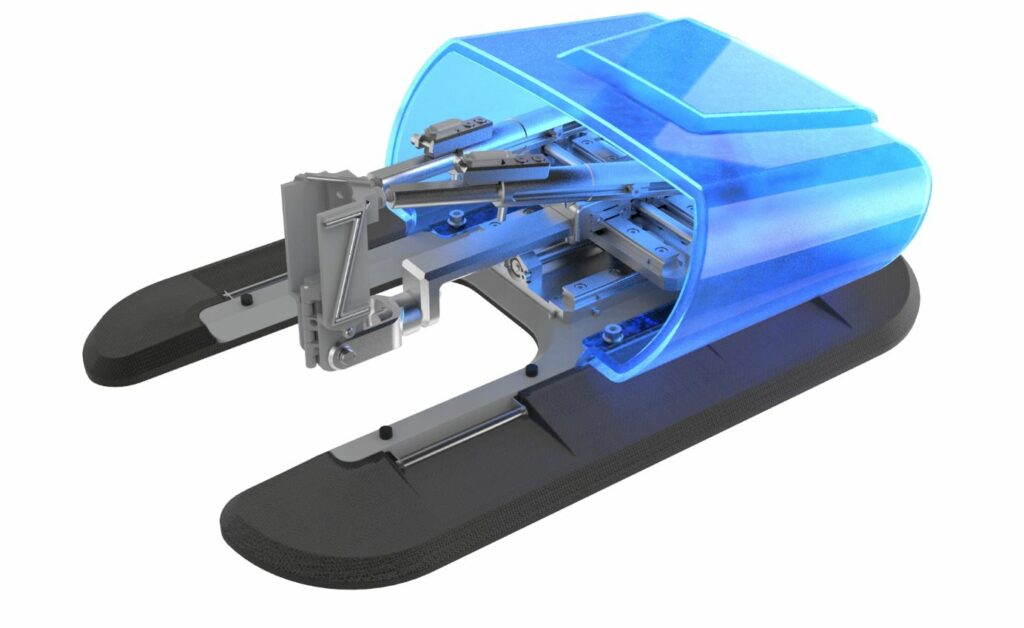
The XACT robotics system is currently approved and being used for CT-guided percutaneous procedures in the abdomen. Later this year, the system’s application is expected to be expanded for use in additional clinical centers and with other imaging modalities, such as Cone-Beam CT and Fluoroscopy, and for additional indications, including spinal and lung procedures.
“Currently, manual navigation of needles for biopsies and needle-based tools for procedures such as abscess drainage and tumor ablation, which are guided by imaging technologies, requires a high level of expertise and presents multiple challenges such as the need to construct a precise needle trajectory in 3D to avoid damaging critical intervening structures while simultaneously needing to compensate for target movement due to patient movements or breathing,” commented Professor Goldberg. “XACT’s solution has thus far demonstrated the potential to successfully addresses these challenges, by integrating robotic needle navigation and steering capabilities to achieve accurate access to a target within the body. We are very pleased with the results from the initial clinical procedures we performed as part of the trial, which further confirm the <1.5mm accuracy findings of the preclinical studies.”
“The CE Mark approval is a key achievement for XACT and an important milestone towards entering the European market,” remarked Chen Levin, CEO of XACT Robotics. “In parallel, the closing of our latest financial round will enable us to initiate the launch of our robotics system next year, while continuing to develop additional features for the system. Our Center of Excellence in Hadassah Medical Center is the first of seven centers that we plan to launch by the end of this year in leading medical centers in the US, Europe and Israel. The goal of these centers is to gain additional clinical experience and to serve as the primary training centers towards the full product launch of the XACT system planned for 2019.”
The CE approved XACT system, which is the only robotics system to integrate robotic navigation with steering capabilities, can be used to accurately access any target in the abdomen in CT-guided percutaneous procedures. The physician plans the procedure on the XACT system by selecting a target and an entry point. The system then recommends a trajectory for approval and for selection of verification checkpoints along the trajectory. Once approved by the physician, the desired tool is attached to the robot allowing it to perform the needle insertion and steering according to the approved trajectory, while the progress is being monitored by the physician. The system actively compensates for breathing and patient movement during the procedure enabling fast, accurate and safe performance of insertion to the desired site of interest. Real-time, closed-loop control coupled with the ability to perform complexangulation 3D trajectories, mean that any deviation from the original planned pathway can be immediately detected and corrected without the need for needle reinsertion or patient repositioning. The XACT system is expected to allow physicians to accurately, consistently, and efficiently reach the desired site of interest in image-guided percutaneous procedures, while significantly reducing the learning curve for physicians performing image-guided percutaneous procedures.
See Full Press Release at the Source: XACT Robotics Receives CE Mark for Its Robotics Navigation System for Percutaneous Procedures
Press Release by: XACT Robotics
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.