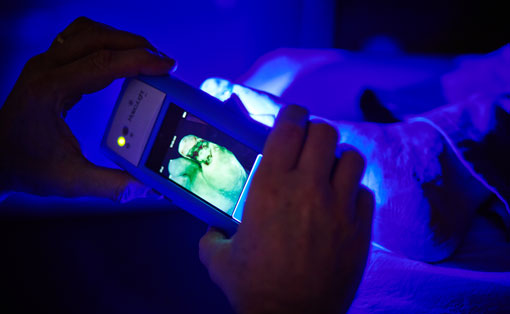
MolecuLight Inc. achieved a major regulatory milestone permitting expansion into the United States market. FDA has granted De Novo clearance for the ground-breaking wound fluorescence imaging device, the MolecuLight i:X. The device digitally captures and documents fluorescence information from wounds and surrounding tissue using still images and videos in real-time. This product is optimized for use at the point-of-care. It is compact and portable, with no requirement to use contrast agents.
“The MolecuLight i:X platform is a significant advancement in the management of chronic wounds, that is already revolutionizing wound care practice in Canada and Europe” said Dr. Ralph DaCosta, Founder, Chief Scientific Officer and Director of MolecuLight Inc. “Thousands of patients to date have already experienced a change in their assessment and treatment by clinicians who feel empowered by the wound fluorescence images they are seeing. As reported in multi-centered published clinical studies, clinicians used the images to inform their wound management practices in real-time, in particular, for guided wound sampling1, cleaning2 and debridement2,3. We’re very excited that US clinicians will soon have the same access to this device as their peers in Canada and Europe.’’
“FDA marketing authorization of the MolecuLight i:X is a monumental milestone for the wound care industry” said Anil Amlani, CEO of MolecuLight Inc. “Thanks to continued clinical studies and 17 publications to date, the clinical evidence is rapidly accumulating that the MolecuLight i:X is a must‑have device in the hands of all wound care clinicians.’’
In the US, the MolecuLight i:X is indicated as a handheld imaging tool that allows clinicians diagnosing and treating skin wounds at the point of care, to (i) view and digitally record images of a wound, and (ii) view and digitally record images of fluorescence emitted from a wound when exposed to an excitation light. The MolecuLight i:X is for prescription use only.
The MolecuLight i:X received Health Canada Medical Device License in 2015 and European CE Mark in 2016.
See Full Press Release at the Source: MolecuLight® Inc.’s first-of-its-kind handheld fluorescence imaging device receives FDA De Novo clearance for US wound care market – MolecuLight
Press Release by MolecuLight
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.