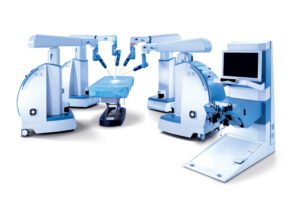
RESEARCH TRIANGLE PARK, N.C.–TransEnterix, Inc. (NYSE American:TRXC), a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery, today announced that the Company has received FDA 510(k) clearance for expanded indications of its Senhance Surgical System. The Company received FDA 510(k) clearance for laparoscopic inguinal hernia and laparoscopic cholecystectomy (gallbladder removal) surgery. There are approximately 760,000 inguinal hernia and 1.2 million laparoscopic cholecystectomy procedures performed annually in the U.S. With this clearance, Senhance System’s total addressable annual procedures in the U.S. has more than doubled to over three million.
“This indication expansion immediately doubles the addressable market for Senhance in the US and validates our regulatory strategy to successfully add to our indications for use,” said Todd M. Pope, president and chief executive officer of TransEnterix. “These expanded procedures are commonly performed at over 95% of hospitals in the United States. We believe this indication expansion will significantly increase the applicability of Senhance to more institutions, particularly those with a busy general surgery practice.”
In the U.S., Senhance is now cleared for laparoscopic colorectal, gynecologic, inguinal hernia and cholecystectomy surgery. This enables Senhance to be used for some of the most common abdominal surgeries, including procedures in general surgery and gynecology.
“We have utilized Senhance broadly across a wide range of general surgery, upper GI surgery and colorectal procedures at our institution,” said Professor Dr. Frank Willeke, Chief of Surgery at St. Marien Hospital in Siegen, Germany. “We believe this procedural expansion for the US will allow surgeons there to incorporate the Senhance, as we have, as a highly-efficient, enabling and very promising technology that can impact the vast majority of surgeries commonly performed by general surgeons and their sub-specialties.”
About TransEnterix
TransEnterix is a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery by addressing the clinical and economic challenges associated with current laparoscopic and robotic options in today’s value-based healthcare environment. The Company is focused on the commercialization of the Senhance Surgical System, which digitizes laparoscopic minimally invasive surgery. The system allows for robotic precision, haptic feedback, surgeon camera control via eye sensing and improved ergonomics while offering responsible economics. The Senhance Surgical System is available for sale in the US, the EU and select other countries. For more information, visit www.transenterix.com.
Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, which are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. Such statements are subject to risks and uncertainties that are often difficult to predict, are beyond our control and which may cause results to differ materially from expectations, including whether the indication expansion will significantly increase the applicability of Senhance to more institutions, particularly those with a busy general surgery practice and whether the procedural expansion for the US will allow US surgeons to incorporate the Senhance as a highly-efficient, enabling and very promising technology that can impact the vast majority of surgeries commonly performed by general surgeons and their sub-specialties. We cannot assure you that our expectations will be realized. For a discussion of the risks and uncertainties associated with TransEnterix’s business, please review our filings with the Securities and Exchange Commission (SEC), including our Annual Report on Form 10-K filed on March 8, 2018 and our other filings we make with the SEC. You are cautioned not to place undue reliance on these forward looking statements, which are based on our expectations as of the date of this press release and speak only as of the origination date of this press release. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
Read More at the Source: TransEnterix Announces FDA Clearance for Expanded Indications for Senhance Surgical System | Business Wire
Courtesy of: Mark Klausner & Joanna Rice
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & development, quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.