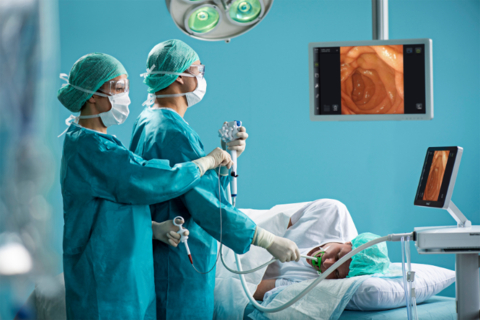
Ambu announces the 510(k) regulatory clearance of the Ambu® aScope™ Gastro and Ambu® aBox™ 2 in the United States. aScope Gastro is Ambu’s first sterile single-use gastroscope and includes new advanced imaging and design features in a combined solution with next-generation display and processor technology. With HD capabilities, the aBox 2 will set a new benchmark in terms of image quality and will be at the centre of Ambu’s endoscopy ecosystem.
Advanced technology to support doctors, health systems, and patients
With the launch of aScope Gastro, Ambu enters the gastroscopy segment, where 20 million procedures take place annually with reusable endoscope systems.
The advanced technology, portability, and cost-effectiveness of Ambu’s solution address the current limitations of reusable endoscopes, and it will be an attractive choice for customers looking to perform gastroscopies across a wide range of care settings (including endoscopy unit, OR, ICU, ER, ASC). Furthermore, the aScope Gastro will support healthcare systems in their efforts to reduce waiting lists and overcome staff shortages, which have been accentuated since the start of the COVID-19 pandemic. Finally, the sterile offering provides a solution to the growing cross-contamination risks, especially for vulnerable patients.
“The Ambu system comes at a time where we’re dealing with waiting lists and staff shortages, and where the ease of setup and elimination of reprocessing, are major advantages. Also, the combination of a sterile single-use gastroscope and a compact display unit opens up the opportunity to expand endoscopy to alternative settings, such as Intensive Care Units,” says Prof. Pradeep Bhandari1, Queen Alexandra Hospital, Portsmouth, UK.
“In the OR setting, having a single-use scope that is immediately available with a small footprint, which requires much less up-front capital outlay than a reusable setup, will be valuable to many hospitals across the country,” says Reginald Bell1, M.D., F.A.C.S, Institute of Esophageal and Reflux Surgery, Lone Tree, Colorado, USA.
With this FDA clearance, Ambu will proceed with commercialization of the aScope Gastro and aBox 2 in the United States.
About Ambu
Ambu has been bringing the solutions of the future to life since 1937. Today, millions of patients and healthcare professionals worldwide depend on the efficiency, safety and performance of our single-use endoscopy, anesthesia, and patient monitoring solutions. We continuously look to the future with a commitment to deliver innovative quality products that have a positive impact on patient care and the work of healthcare professionals. Headquartered near Copenhagen in Denmark, Ambu employs approximately 4,500 people in Europe, North America and the Asia Pacific. For more information, please visit ambu.com or ambuUSA.com or follow us on our corporate LinkedIn and USA LinkedIn pages.
See Full Press Release at the Source: Ambu Announces FDA Clearance of Single-Use Gastroscope and Next-Generation Display Unit
Press Release by: Ambu
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.