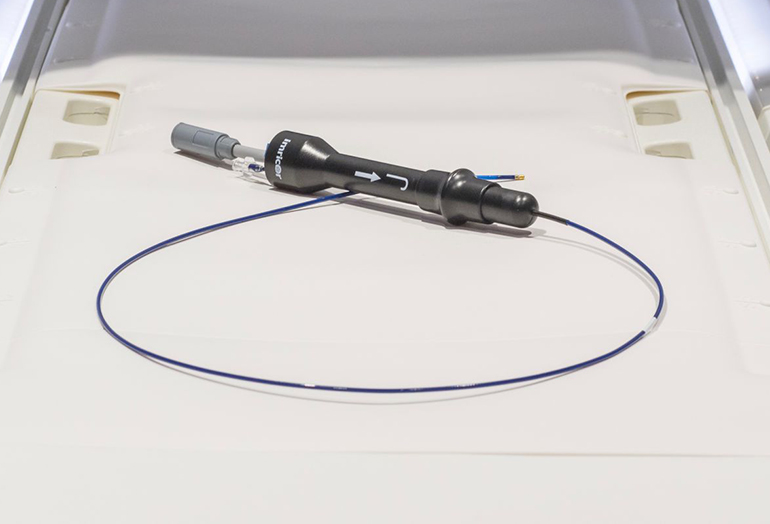
Imricor Medical Systems, Inc. (ASX:IMR) today announces that it has received CE mark approval for its Vision-MR Ablation Catheter and Vision-MR Dispersive Electrode. This follows earlier CE mark approval for Imricor to place its Advantage-MR EP Recorder/Stimulator System on the market in Europe. Imricor is the first and only company to offer cardiac ablation devices for use in the MRI environment.
Imricor Chairman and CEO, Steve Wedan said, “We are absolutely thrilled to bring to market our products for performing cardiac catheter ablations guided by real-time MRI.
“Receipt of CE mark approval marks a major accomplishment for the Imricor team, as we pursue our mission to improve the lives of people worldwide by delivering devices that enable iCMR procedures.”
The Vision-MR Ablation Catheter is the Company’s prime product offering, specifically designed to work under real-time MRI guidance, with the intent of enabling higher success rates along with a faster and safer treatment compared to conventional procedures using x-ray guided catheters.
“This approval opens the door for patients to benefit from this exclusive combination of therapy and imaging. We are extremely excited to offer this to our patients and to lead the way forward with this new approach,” said Professor Gerhard Hindricks MD, Head of the Department of Electrophysiology at the Leipzig Heart Center.
Imricor sells its capital and consumable products to hospitals and clinics for use in Interventional Cardiac Magnetic Resonance Imaging (iCMR) labs, in which ablation procedures using the Vision-MR Ablation Catheter can be performed.
Wedan stated, “With inventory located in our European warehouse and a well-established roll out plan in place, we will now move swiftly to the execution of a controlled commercial launch to ensure smooth adoption and good clinical outcomes.”
Imricor has entered into agreements with multiple sites regarding the sale of its products. These sites have Advantage-MR systems and are ready to commence procedures having achieved CE mark approval.
Imricor Director of Marketing, Nick Twohy stated, “The future of cardiac ablation therapy is in MR-guidance and this product launch is the first step towards a new standard of care. We look forward to partnering with healthcare professionals to offer a new treatment option for patients who suffer from arrhythmias.”
See Full Press Release: Vision-MR Ablation Catheter Receives CE Mark | Business Wire
Written by: MRICOR MEDICAL SYSTEMS, INC.

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.