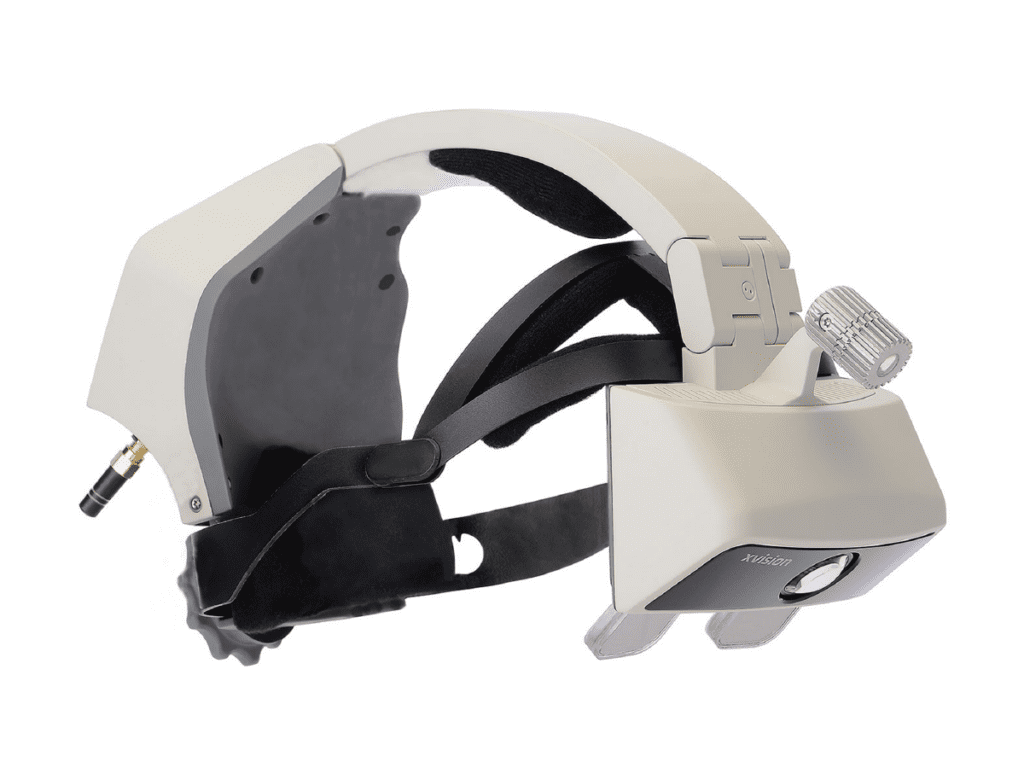
Augmedics, a pioneer in augmented reality surgical image guidance, has announced U.S. Food and Drug Administration (FDA) 510(k) clearance and the U.S. launch of its groundbreaking xvision Spine system (XVS), the first AR guidance system to be used in surgery. xvision Spine allows surgeons to visualize the 3D spinal anatomy of a patient during surgery as if they had “x-ray vision,” and to accurately navigate instruments and implants while looking directly at the patient, rather than a remote screen.
The xvision consists of a transparent near-eye-display headset and all elements of a traditional navigation system. It accurately determines the position of surgical tools, in real time, and a virtual trajectory is then superimposed on the patient’s CT data. The 3D navigation data is then projected onto the surgeon’s retina using the headset, allowing him or her to simultaneously look at the patient and see the navigation data without averting his or her eyes to a remote screen during the procedure. The system is designed to revolutionize how surgery is done by giving the surgeon better control and visualization, which may lead to easier, faster and safer surgeries.
Augmedics successfully completed a percutaneous laboratory study with the xvision Spine at Rush University Medical Center with investigators Frank Phillips, M.D., Camilo Molina, M.D., Kornelis Poelstra, M.D., Ph.D., Larry Khoo, M.D., and Matthew Colman, M.D. Ninety-three screws were positioned in the thoracic and sacro-lumbar areas of five different cadavers. The study was conducted as evidence to the FDA to evaluate the accuracy of the xvision Spine system by comparing the actual screw tip position and trajectory versus the virtual. The result of overall clinical accuracy, analyzed by two independent neuro-radiologists, was 98.9 percent using the Heary (thoracic) and Gertzbein (lumbar) scales. This study adds to the evidence of accuracy and usability found last year in another cadaver study performed by two surgeons from Johns Hopkins Medicine, Daniel Sciubba, M.D., and Timothy Witham, M.D., one surgeon from Sheba Tel-Hashomer, Israel, Ran Harel, M.D., and one from Assaf Harofeh, Israel, Yigal Mirovsky. The study last year was conducted at Vista Labs, an independent lab in Baltimore, with results published in the Journal of Neurosurgery: Spine.
“The ability that Augmedics’ xvision provides to visualize the patient’s spinal anatomy in 3D, coupled with live CT images as a retina display, is game changing,” said Frank Phillips, M.D., Professor of Orthopaedic Surgery, Rush University Medical Center. “The efficiency and accuracy this augmented reality technology enables in placing spinal implants without looking away from the surgical field – as well as the ability to “see the spine” through the skin in minimally invasive procedures – differentiates the xvision from conventional spinal navigation platforms. The economics of the xvision system are also compelling in both the hospital and the surgicenter environment.”
“Augmedics’ mission is to give surgeons more control by creating technological advances that cater to their needs and fit within their workflow,” said Nissan Elimelech, founder and CEO of Augmedics. “xvision is our first product of many to follow that will revolutionize surgery, as it gives surgeons the information they need, directly within their working field of sight, to instill technological confidence in the surgical workflow and help them do their jobs as effectively and safely as possible.”
xvision is now available for sale in the United States, with headset distribution expected to begin in early 2020. Augmedics plans to explore additional surgical applications for xvision beyond spinal surgery. The system’s small footprint, economical cost and compatibility with current instrumentation is designed to allow easy integration into any surgical facility nationwide.
See Full Press Release: Augmedics Announces FDA 510K Clearance and U.S. Launch of xvision™, the First Augmented Reality Guidance System for Surgery | Business Wire
Written by: Augmedics

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.